Two gene therapies were recently approved by the Food and Drug Administration (FDA) to treat sickle cell disease: Casegevy from CRISPR/Vertex and Lyfgenia from bluebird bio. You probably have questions about these new treatment options. Read more below.
Is gene therapy a cure for sickle cell disease?
Gene therapy is a potentially curative therapy. This means that it could act as a cure, but it is too new to say for sure. It causes a big decline in pain episodes, but we need to learn more about long-term impacts and side effects. It is also not a “one-and-done” treatment. The FDA currently recommends 15 years of patient follow up.
How does gene therapy work?
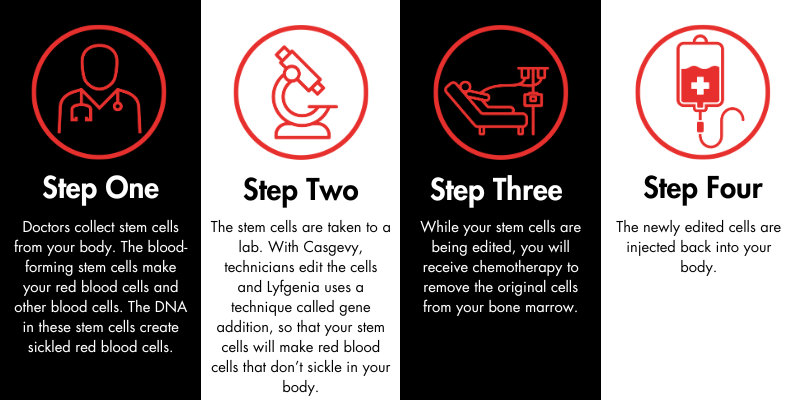
When will it be available?
Likely in early 2024.
Am I eligible for gene therapy?
Casgevy and Lyfgenia are approved for people ages 12 and up. Sickle cell disease SS and S-beta-zero-thalassemia are eligible. The FDA indicates that sickle cell disease SC is not included. Additionally, you may also not be able to receive gene therapy if you have:
- A recurring viral infection
- Significant organ damage
Additionally, if you have a matched sibling, you should go down the path of a matched-sibling-donor bone marrow transplant instead of gene therapy. Talk to your doctor about this option.
What are the side effects?
Gene therapy requires you to have chemotherapy. This means it could result in:
- Infertility or secondary cancer
- Temporary weakening of the immune system so that you cannot fight off any infections
- Temporary hair loss
Where can I receive gene therapy?
Treatment will likely be at an existing bone marrow transplant center that also works with sickle cell disease experts. These may be hard to find. SCDAA will be providing a list of facilities, once identified, on our website: sicklecelldisease.org.
How much will it cost? Will insurance cover it?
Gene therapy is expensive, and FDA-approved high-cost medications can come with barriers. Casgevy is estimated to cost $2.2 million, and Lyfgenia is estimated to cost $3.1 million. We are still waiting to hear how insurance companies will handle gene therapies.
Does gene therapy work for all types of SCD?
As far as we know, yes. It is designed to be able to help raise fetal hemoglobin (HbF), which should work for all different kinds of sickle cell disease. However, the amount of experience with the different kinds has not been nearly the same – we know the most for SS and S Beta zero thalassemia types.
Are we the first community to receive gene therapy?
Casgevy is the first approved use of gene editing. However, gene addition therapy has been used to treat other conditions, including:
- Retinal degeneration
- Spinal muscular atrophy
- Beta-thalassemia
- X-linked Adrenoleukodystrophy
- Hemophilia A & B
- Bladder cancer
- Acute-lymphoblastic leukemia
To learn more about the gene therapies used to treat these conditions, click here.
For a longer (but not complete) list of conditions that have been treated using gene therapy, click here.
Is it safe? How do I know if this is right for me?
For many people, the benefits of this new treatment outweigh the risks. Your doctors will help you determine whether this is a good option for you.
What questions should I ask my doctor?
- How long will this take?
- What is the time commitment?
- Where is the nearest treatment center?
- What are my other options?
How do I learn more about gene therapy?
There are several resources available. The below sources are considered trustworthy and non-biased by SCDAA.
- The Democratizing Education for Sickle Cell Gene Therapy Project created materials with input from individuals with SCD and their families. Read their FAQs and visit their website.
- SCDAA has produced several videos on gene therapy. Watch the gene therapy masterclass and this webinar to learn more.
- OneSCDVoice hosts gene therapy resources on their Gene Therapy 101 page.
- The Dec. 8 FDA approval statement for Casgevy and Lyfgenia.
To learn more about Vertex’s Casgevy, visit casgevy.com. To learn more about bluebird bio’s Lyfgenia, visit my bluebird support.
We encourage you to subscribe to our email list for news and updates.
Updated Dec. 14, 2023, at 11:09 a.m. EST
Please note: A previous version of this FAQ incorrectly stated that Casgevy is approved for people ages 12 and up and Lyfgenia is approved for those ages 12 to 50. This statement has been corrected to note that both Casgevy and Lyfgenia are approved for people ages 12 and up.





 DONATE NOW
DONATE NOW