SiNERGe and Sickle Cell Disease Association of America, Inc. (SCDAA) are collaborating with sickle cell advocacy groups, community-based organizations, hospitals, governments and other key stakeholders in the sickle cell community on Shine the Light on Sickle Cell, a 24-hour awareness campaign to celebrate the 10th anniversary of World Sickle Cell Awareness Day on June 19, 2019.
June 19th was officially designated by the United Nations as World Sickle Cell Awareness Day. The international awareness day is observed annually with the goal to increase public knowledge and an understanding of sickle cell disease (SCD) and sickle cell trait (SCT), and the challenges experienced by patients and their families and caregivers. On June 19th and within the month of June, individuals and organizations are hosting a number of activities across the country.
“June 19 is a day of unity and purpose for the sickle cell community,” says Dr. Sophie Lanzkron, associate professor of medicine at the Johns Hopkins University School of Medicine and co-director of the SiNERGe collaborative. “We’re proud to work with the SCDAA to highlight the need for increased awareness about sickle cell disease and its impact on individuals and their families here in the U.S. and abroad.”
SCD is a global health problem affecting millions of people around the world. It is estimated that approximately 100,000 Americans have the disease, and more than 1,000,000 worldwide have sickle cell trait. Each year, approximately 1,000 babies in the United States are born with SCD, and there is no universal cure for this life-threatening disease.
“SCDAA is proud to collaborate with SiNERGe on this important national awareness campaign that has brought together the diverse stakeholders within the sickle cell community to create events, activities and opportunities to bring attention to sickle cell disease and to encourage individuals to get involved in our efforts to advocate on behalf of the those affected by this disease,” said SCDAA President and CEO Beverley Francis-Gibson.
Visit the Shine the Light on Sickle Cell Campaign Facebook page at www.facebook/com/pg/ShineTheLightOnSickleCell/events to learn about the events happening across the country on June 19 and in the month of June.
Click here to read more!
Author Archives: admin
GBT Awards More than $200,000 in Grants to Five Nonprofit Organizations through New ACCEL Program Aimed at Improving Access to Healthcare for People Living with Sickle Cell Disease
Innovative Healthcare Programs for Sickle Cell Community
On June 10, 2019, Global Blood Therapeutics, Inc. (GBT) (NASDAQ: GBT) announced that five nonprofit organizations have been awarded more than $200,000 in grants through the company’s new Access to Excellent Care for Sickle Cell Patients Pilot Program (ACCEL).
Five grant recipients – the Center for Comprehensive Care and Diagnosis of Inherited Blood Disorders (CIBD) and the Sickle Cell Disease Foundation (SCDF), the James R. Clark Memorial Sickle Cell Foundation, The Johns Hopkins University School of Medicine, the MAVEN Project (Medical Alumni Volunteer Expert Network) and the Sickle Cell Foundation of Georgia – will each receive grants to accelerate the development of promising programs that have the potential over time to deliver high-quality healthcare to people living with sickle cell disease (SCD).
“We created the ACCEL program to help address the significant challenges that people living with SCD face every day in accessing quality healthcare in their communities. One solution to addressing these inequities is to encourage non-profit organizations to develop innovative programs that can ensure more children and adults living with SCD get access to high-quality care,” said Jung E. Choi, chief business and strategy officer, and head of patient advocacy and government affairs at GBT. “We are thankful to the many applicants who submitted compelling proposals.”
Click here to read more!
Congressional Briefing on Progress in Sickle Cell Disease Treatment & Policy Implications
TUESDAY, JUNE 18, 2019 | 2:30 – 3:30 PM
DIRKSEN SENATE OFFICE BUILDING, ROOM G50
RSVP: Betsy Foss-Campbell, bfoss@asgct.org
Join us for updates on Gene therapy approaches presented by prominent scientists in the field
How policymakers can support the development of treatment options for sickle cell disease
SPEAKERS
Tim Scott, United States Senator
Francesca Cook, MPH, Government Relations Committee member, ASGCT; Senior Director, Pricing and Market Access, REGENXBIO
Julie Kanter, MD, Director of Adult Sickle Cell Program; Associate Professor of Hematology and Oncology, University of Alabama at Birmingham
Punam Malik, MD, Professor of Pediatrics, Marjorie Johnson Chair of Gene and Cell Therapy, Director, Cincinnati Comprehensive Sickle Cell Center; Cancer and Blood Disease Institute, Cincinnati Children’s Hospital
Rodrick Murray, Patient advocate providing a personal story on gene therapy’s value for treating sickle cell disease
Matthew Porteus, MD, PhD, Professor of Pediatrics (Stem Cell Transplantation), Stanford University
David Williams, MD, Senior Vice President and Chief Scientific Officer, Boston Children’s Hospital; Chief, Hematology/Oncology, Boston Children’s Hospital; President, Dana-Farber/Boston Children’s Cancer and Blood Disorders Center; Leland Fikes Professor of Pediatrics, Harvard Medical School
How can policymakers help support the development of gene therapies for sickle cell disease?
- Support the need for robust NIH research funding
- Support appropriations to the sickle cell disease prevention and treatment program and implementation of new surveillance and screening authorities passed in 2018
- Enable novel payment models for approved gene therapies
Hosted in partnership with the Pediatric Hospital Sickle Cell Disease Collaborative and

National Family, Patient, Public Sector, and Multi-employer Groups Join Effort to Protect Health Care Coverage
The Alliance to Fight the 40 | Don’t Tax My Health Care, a broad-based coalition committed to repealing the 40 percent tax on employer-provided health benefits welcomes Families USA, Public Sector HealthCare Roundtable, the National Coordinating Committee for Multi-employer Plans (NCCMP), and the Sickle Cell Disease Association of America in the effort to protect the health care coverage upon which more than half of all Americans depend.
Click here to read more.
Jordin Sparks Discusses the Emotional and Social Impact of Sickle Cell Disease
Jordin Sparks is a Grammy-nominated, multi-platinum recording artist, but she also wants to spark a conversation about the emotional and social impact of sickle cell disease.
Sickle Cell Disease Association of America, Inc. thanks Jordin Sparks, for your commitment to increasing awareness about sickle cell disease! Click here to read an article featuring Generation S spokesperson, Jordin Sparks.
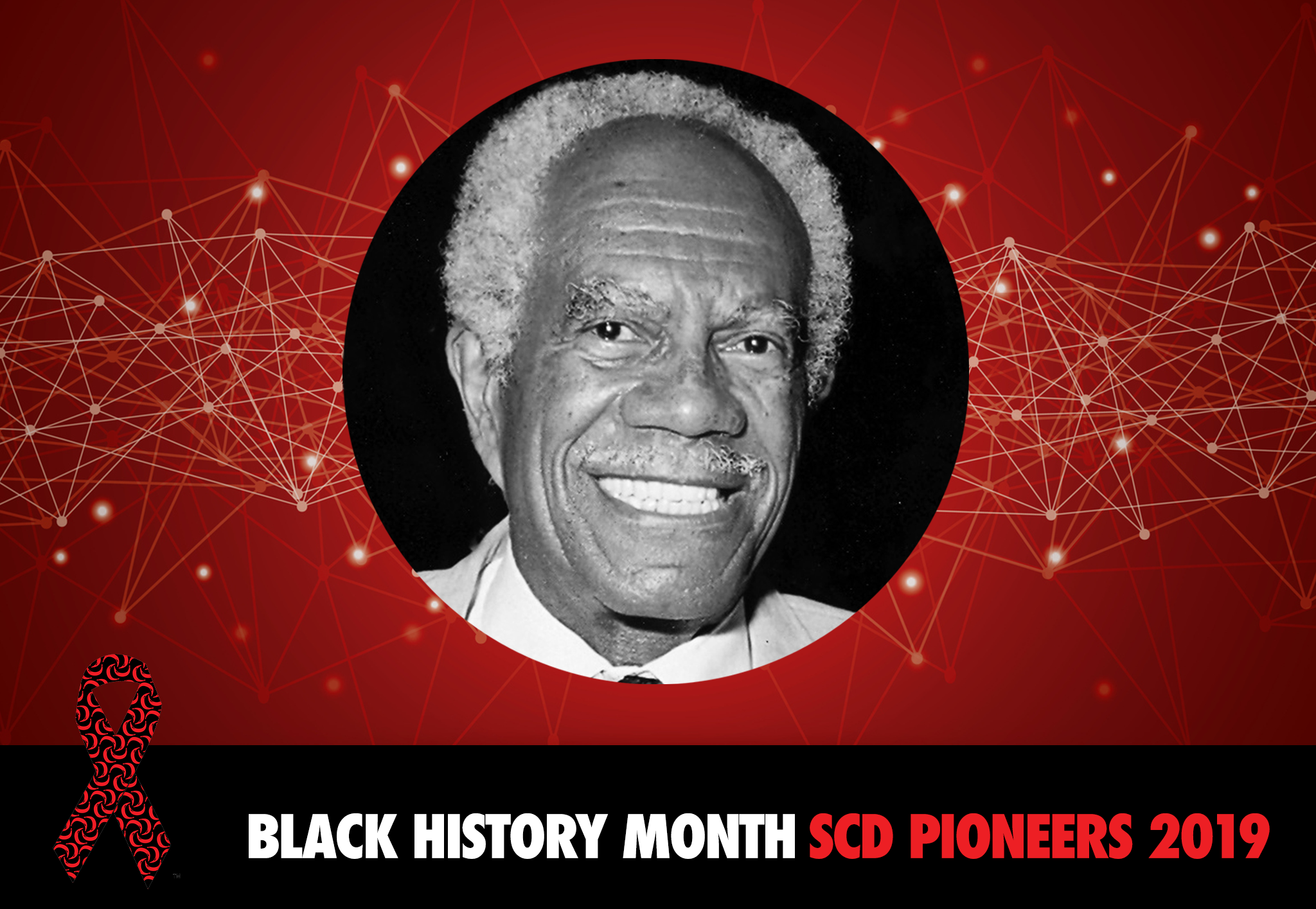
Charles F. Whitten: Black History Month SCD Pioneers 2019
Dr. Charles F. Whitten: A Physician. Medical pioneer. Founder and President Emeritus of the Sickle Cell Disease Association of America, Inc.
Dr. Charles F. Whitten was a physician, a medical pioneer and the founder and president emeritus of the Sickle Cell Disease Association of America, Inc. (SCDAA). His dedication and commitment to SCDAA and to those with sickle cell disease will be forever remembered and cherished. Dr. Whitten passed away on August 14, 2008 at the age of 86.
Dr. Whitten was born on February 2, 1922 in Wilmington, Delaware to school teachers Emma Clorinda Carr Whitten and Tobias Emmanuel Whitten. In 1942, he earned his B.S. degree in zoology from the University of Pennsylvania. He then studied medicine at Meharry Medical College in Nashville, Tennessee and earned his M.D. degree in 1945 at age twenty-three.
Dr. Whitten worked as a general practitioner in Lackawanna, New York from 1946 to 1951, and then served two years as a captain in the US Medical Corps before returning to the University of Pennsylvania’s Graduate School of Medicine for a year of advanced study in pediatrics. In 1953, he began a two-year residency in pediatrics at Children’s Hospital in Buffalo, NY. In 1955, he moved to Detroit, MI for a one-year fellowship to study pediatric hematology under Dr. Wolf Zeltzer.
In 1957, he joined the faculty of Wayne State University School of Medicine and was subsequently appointed chief of pediatrics at the Detroit Receiving Hospital, making him the first African American to head a hospital department in Michigan. During his tenure at Wayne State University Dr.
Whitten served for 16 years as Associate Dean for Curricular Affairs, and 10 years as Dean for Special Programs. He authored over 100 journal articles and 7 book chapters.
Dr. Whitten was deeply concerned about the under representation of African American physicians. In 1969, this inspired him to conceptualize and implement an innovative post-baccalaureate enrichment program, the first initiative of its kind in the nation. By its 30th anniversary, the program had graduated almost 300 students of color, more than any other medical school with the exception of Howard University and Meharry College of Medicine. His model has been replicated in medical schools across the country.
Dr. Whitten is widely regarded in the medical community as a trailblazer for his work in sickle cell disease screening. In 1971, he formed the Sickle Cell Detection and Information Center in Detroit, MI, a comprehensive community program which developed educational tools for teaching children and families about sickle cell disease. He directed this center for 19 years. He also founded the Sickle Cell Disease Association of America of Michigan (SCDAAMI) based in Detroit and served as its President until his death. SCDAAMI remains one of the original members of SCDAA. Understanding the need for a national agenda for sickle cell disease, Dr Whitten was instrumental in the creation of the National Association for Sickle Cell Disease, now known as the Sickle Cell Disease Association of America, Inc.
In 2002, Whitten was named Michiganian of the Year, and in 2004, he was named distinguished professor of pediatrics, emeritus at Wayne State University. The Black Medical Association established the Charles F. Whitten Lifetime Achievement Award, which is presented annually. The first award went to Dr. Whitten. He also has been honored with a Special Recognition Award from the Association of American Medical Colleges for his pioneering efforts in medical education and treatment. In addition, SCDAA presented him with a Legacy Award for his 21 years of service in the organization’s leadership, and has since named a special lecture after him at its Annual National Convention.
SCDAA celebrates the life and achievements of Dr. Charles F. Whitten for his outstanding commitment and dedication to the sickle cell community. We cannot thank him enough for his leadership and for inspiring generations to be advocates and leaders in the sickle cell community.
(Information for this article provided by Historymakers.org, Findagrave.com and wayne.med.edu)
GBT Launches ACCEL Grants Program to Improve Access to Care for People with Sickle Cell Disease
—The Access to Excellent Care for Sickle Cell Patients Pilot Program (ACCEL) Supports Novel Projects Aimed at Improving Access to High-Quality Healthcare for People with Sickle Cell Disease—
—GBT Will Fund Proposals With the Highest Potential to Impact Patient Care—

SOUTH SAN FRANCISCO, Calif. – Feb. 19, 2019 – Global Blood Therapeutics, Inc. (GBT) (NASDAQ: GBT) today announced the launch of the Access to Excellent Care for Sickle Cell Patients Pilot Program (ACCEL) to provide grant funding to support novel projects aimed at improving access to high-quality healthcare for sickle cell patients in the United States.
GBT will fund as many as three proposals up to $50,000 each to accelerate the development of promising programs with the potential over time to deliver high-quality healthcare to people living with sickle cell disease (SCD).
“Studies show that healthcare delivery to people living with SCD is typically suboptimal. For example, in the United States, fewer than 10 percent of Medicaid and Medicare patients living with SCD see a hematologist at least once per year and approximately 20 percent of SCD patients receive most of their care in the emergency room,” said Jung Choi, who oversees patient advocacy and government affairs at GBT. “We are excited to launch ACCEL to encourage the development of innovative solutions to provide underserved SCD patients with better access to high-quality care and support.”
ACCEL builds on discussions from the SCD Access to Care Summit sponsored by GBT and held in September 2018, during which healthcare providers and members of the sickle cell community discussed programs that are successfully working to address the significant gaps in healthcare delivery for both adults and children living with SCD. During the Summit, participants created draft roadmaps of these models to help disseminate best practices and to encourage the initiation of new SCD access-to-care programs.
Proposals will be reviewed by a panel of GBT personnel and external stakeholders with expertise in the issues affecting people with SCD. The panel will select as many as three proposals based on strength, degree of innovation and highest potential impact to patient care.
For more information about ACCEL, visit https://www.gbt.com/patients/accel-grant-program/ or email patientadvocacy@gbt.com.
About Sickle Cell Disease SCD is a lifelong inherited blood disorder caused by a genetic mutation in the beta-chain of hemoglobin, which leads to the formation of abnormal hemoglobin known as sickle hemoglobin (HbS). In its deoxygenated state, HbS has a propensity to polymerize, or bind together, forming long, rigid rods within a red blood cell (RBC). The polymer rods deform RBCs to assume a sickled shape and to become inflexible, which causes hemolytic anemia (low hemoglobin due to RBC destruction) that can lead to multi-organ damage and early death. This sickling process also causes blockage in capillaries and small blood vessels. Beginning in childhood, SCD patients typically suffer unpredictable and recurrent episodes or crises of severe pain due to blocked blood flow to organs, which often lead to psychosocial and physical disabilities.
About Global Blood Therapeutics
GBT is a clinical-stage biopharmaceutical company determined to discover, develop and deliver innovative treatments that provide hope to underserved patient communities. GBT is developing two therapies for the potential treatment of sickle cell disease, including its late-stage product candidate, voxelotor, as an oral, once-daily therapy. To learn more, please visit www.gbt.com and follow the company on Twitter @GBT_news.
Dr. Doris Wethers Blazed a Trail for Newborn Testing for Sickle Cell Disease
 Sickle Cell Disease Association of America, Inc. (SCDAA) celebrates the life and work of Dr. Doris L. Wethers who died at the age of 91 on January 28, 2019.
Sickle Cell Disease Association of America, Inc. (SCDAA) celebrates the life and work of Dr. Doris L. Wethers who died at the age of 91 on January 28, 2019.
Emmaus Life Sciences Launches Its Commercial Co-Payment Assistance Program for Endari™
TORRANCE, Calif., Jan. 28, 2019 /PRNewswire/ — Emmaus Life Sciences, Inc. (Emmaus), a leader in sickle cell disease treatment, announced today that it will provide financial assistance to help eligible patients afford their monthly co-payment for Endari™ (L-glutamine oral powder) [1] . The program is limited to financially eligible patients covered by commercial insurance.
Mark Diamond, Emmaus’ Vice President of Commercialization, commented: “We are committed to removing barriers between patients and Endari – giving a greater number of patients access to our effective treatment for sickle cell disease.”
Click here to read the full press release.
Sickle Cell Disease Association of America, Inc. (SCDAA) in no way endorses any medications, treatments, clinical trials, or studies reported through Get Connected. Information is provided to keep the readers informed. Because the manifestations and severity of sickle cell vary among individuals, personalized medical management is essential. Therefore, it is strongly recommended that all drugs and treatments be discussed with the reader’s physician(s) for proper evaluation and treatment.
CRISPR Therapeutics and Vertex Announce FDA Fast Track Designation for CTX001 for the Treatment of Sickle Cell Disease
CRISPR Therapeutics and Vertex Pharmaceuticals Incorporated today announced that the U.S. Food and Drug Administration (FDA) has granted Fast Track Designation for CTX001 for the treatment of sickle cell disease (SCD). CTX001 is an investigational, autologous, gene-edited hematopoietic stem cell therapy for patients suffering from severe hemoglobinopathies.
The FDA’s Fast Track program is designed to facilitate the development and expedite the review of drugs to treat serious conditions and fill an unmet medical need. A drug granted Fast Track Designation may be eligible for several benefits, including more frequent meetings and communications with the FDA and, if relevant criteria are met, the potential for Accelerated Approval, Priority Review or Rolling Review of a Biologics License Application (BLA).
In October 2018, CRISPR and Vertex announced the FDA acceptance of the Investigational New Drug application (IND) for CTX001 for the treatment of SCD, and enrollment in a Phase 1/2 trial in SCD is currently underway in the U.S. The companies are also evaluating CTX001 for the treatment of β-thalassemia, and enrollment in a Phase 1/2 trial in β-thalassemia is currently open at multiple clinical trial sites in Europe.
Click here to read the press release issued today.