On Dec. 8, 2023, the Food and Drug Administration (FDA) approved exagamglogene autotemcel (exa-cel) gene therapy for sickle cell disease (SCD), making it the first treatment of its kind available to individuals with SCD in the United States. The approval of this potentially curative therapy marks a major advance in the treatment of sickle cell disease; however, there are valid concerns about accessibility and the potential for adverse effects.
“Exa-cel marks a big step forward for sickle cell disease research and treatment,” said Dr. Lewis Hsu, chief medical officer of the association. “The results of the clinical trial are very impressive. The patients selected had a lot of pain (two or more vaso-occlusive crisis pain hospitalizations per year for two years). The data shows that, after exa-cel was administered, 29 out of 30 (96.7%) patients were free of vaso-occlusive crises for at least nine consecutive months. The patients’ health-related quality of life also improved in every way: physically, emotionally, socially and functionally.”
“Exa-cel is an exciting and potentially curative addition to the treatments available to sickle cell warriors,” said Regina Harfield, president and CEO of the association. “This is a historic milestone, but everyone may not be eligible for exa-cel therapy. We must continue to move forward with research to ensure that there is a solution for every member of our community.”
What is exa-cel, and how does it work?
Exa-cel (formerly CTX001) is a genetic therapy that uses CRISPR-Cas9 to disrupt the BCL11A gene in red blood cell precursors in the bone marrow. The BCL11A gene’s function is to tell red blood cells to stop producing fetal hemoglobin after birth. Exa-cel therapy locates the gene and cuts into it, effectively “turning it off.” As a result, red blood cells will have fetal hemoglobin again, which reduces the damage from sickle hemoglobin.
Is this a cure for sickle cell disease?
SCDAA recognizes exa-cel as a “potentially curative” therapy. The treatment is so new that more data is needed to understand its impact and patient prognosis. Additionally, the word “cure” suggests a simple solution that does not reflect the reality of this therapy. Even after completing treatment, the FDA recommends 15 years of patient monitoring for health issues.
What is the treatment like?
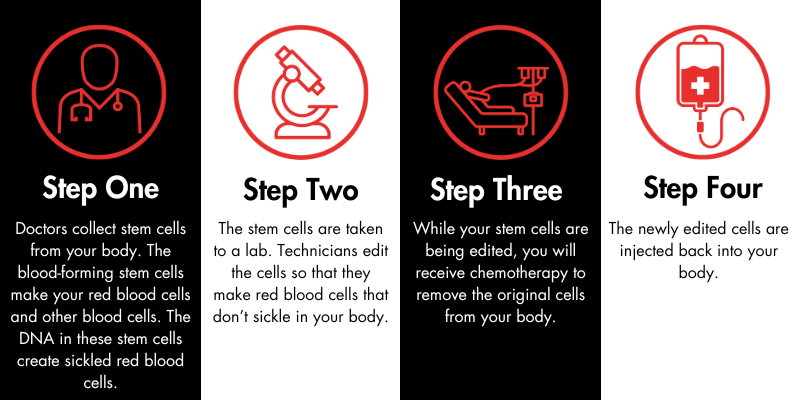
Exa-cel is administered during a one-time infusion; however, there are steps that patients must take to prepare for the treatment. First, the patient’s care team will collect stem cells, which are the progenitors of red blood cells, from their body. Then, those cells will be edited in a lab. The patient will undergo chemotherapy to remove the original, abnormal stem cells from the body. After this process is complete, the treated stem cells are injected back into the patient through an intravenous process like a transfusion (not surgery). The whole procedure takes about a year. It is similar to autologous bone marrow transplantation, as there is no need to find a donor of stem cells.
How effective is it?
Exa-cel provides a significant reduction in acute episodes of sickle cell pain within a few years of administration. More years of follow-up will be needed to determine whether exa-cel will also reduce the organ damage of sickle cell disease and if the stem cells treated with exa-cel continue to produce non-sickling red blood cells for the rest of the person’s life, or if the stem cells die off over a certain number of years. Currently the treatment requires chemotherapy, which means there are also concerns about chemotherapy-associated complications, such as infertility or secondary cancer.
When will it be available for use?
Exa-cel will likely be available in early 2024.
Who is eligible?
It is approved for use in individuals ages 12 and up. At this time, we are unsure of what the prerequisites will be for gene therapy. Additionally, a portion of the SCD population will not be eligible for the treatment, including individuals with HBSC, which represents a third of the SCD U.S. population.
Where is gene therapy administered?
Individuals with SCD can receive gene therapy at existing bone marrow treatment facilities with sickle cell expertise which may pose accessibility issues to patients. SCDAA encourages gene therapy centers to partner with sickle cell centers, such as in the National Alliance of Sickle Centers, so that there is expertise to monitor for sickle cell organ damage.
How much will it cost? Will insurance cover gene therapy?
Gene therapy treatments are produced through expensive, highly technical processes. The cost estimates for treatment are $2 million and up. The high price may be worthwhile as lifelong care may exceed the one-time cost of gene therapy. FDA-approved high-cost medications come with insurance barriers and rules that are not evidence-based.
What does this approval mean for people living with sickle cell?
This approval is expected to be life-changing for many and ushers in a new age of treatment for sickle cell disease. Until now, the only way to cure sickle cell disease was through a bone marrow transplant, which is not a widely accessible option because it requires a matched bone marrow donor. Gene therapy does not require a donor; therefore, it has the potential to be a more widely available treatment.
What does it mean for sickle cell treatment in the future?
Exa-cel is the first gene therapy treatment approved by the FDA for sickle cell disease. It opens the door for other gene therapies to gain approval and helps advance research into other potentially curative treatments. At the same time, there are concerns that this approval will create an increase in competition for health care resources that could make it difficult to access other forms of treatment outside of gene therapy. Many people will not be eligible to receive exa-cel and/or other forms of gene therapy. To provide the highest quality of care to these individuals, we need to continue research into a variety of treatment options beyond gene therapy.
What are the implications for the medical community at large?
More broadly, exa-cel is the first FDA-approved CRISPR therapy for a genetic disease. This could have wide-reaching impacts for individuals with other conditions like cystic fibrosis, Tay-Sachs disease and others.
Where can I find more information?
Research into exa-cel specifically is ongoing; however, there is a wide variety of information about gene therapy for sickle cell disease available as it relates to clinical trials.





 DONATE NOW
DONATE NOW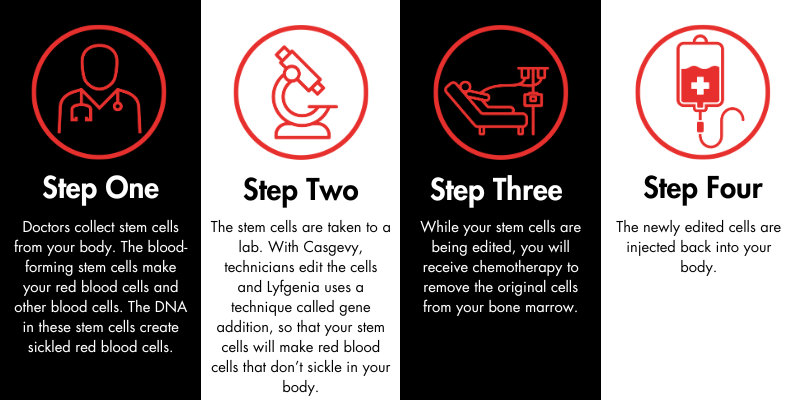

 The Sickle Cell Disease Association of America, Inc., (SCDAA) is saddened to hear the news of the passing of Dr. Lennette Benjamin. Dr. Benjamin was a trailblazing physician who made many outstanding contributions to the sickle cell community. She was one of the first to establish a “day hospital” as an alternative to the emergency room for pain management – an approach that is today recognized as a best practice in care.
The Sickle Cell Disease Association of America, Inc., (SCDAA) is saddened to hear the news of the passing of Dr. Lennette Benjamin. Dr. Benjamin was a trailblazing physician who made many outstanding contributions to the sickle cell community. She was one of the first to establish a “day hospital” as an alternative to the emergency room for pain management – an approach that is today recognized as a best practice in care.