NEW RESOURCE AVAILABLE: This tool from the National Alliance of Sickle Cell Centers can help sickle cell providers make important decisions about dosage when prescribing opiates. The calculator was developed was Paula Tanabe, RN, Ph.D., and Patricia Kavanagh, M.D., through a grant funded by the National Heart, Lung and Blood Institute. Click here to access the resource.
Category Archives: Uncategorized
SCDAA and MedicAlert Foundation Launch Pilot Program
Leading Sickle Cell Organization and MedicAlert Foundation Launch Pilot Program to Improve Emergency Care During Sickle Cell Pain Crises
Non-profits partner to help people with sickle cell disease get faster, better emergency care.
HANOVER, MD – Sickle Cell Disease Association of America (SCDAA), the leading national organization and voice for people with sickle cell disease (SCD), today launched a pilot program with MedicAlert Foundation to enhance the safety and well-being of people living with sickle cell disease (SCD).
Acute pain episodes known as sickle cell crises are one of the most common and debilitating symptoms of SCD. These crises can be unpredictable and extremely painful, lasting from a few hours to a few weeks. They’re the number one reason people with SCD seek emergency treatment.
However, SCD patients seeking treatment for a sickle cell crisis face hurdles to getting the care they need in the Emergency Department (ED). The goal of the pilot program is to improve access to timely, effective emergency care for people experiencing a sickle cell crisis.
The program provides participants with a MedicAlert digital health profile to store their physician-approved pain plan, physician contact information, and other vital health data. Each participant also receives a customized Smart Medical ID Card, which provides easy access to their health information and pain plan via a QR code.
The goal is to shortcut time to diagnosis and treatment. When seeking emergency treatment, participants will present their Smart ID Card to share their health history with ED personnel – confirming their SCD status, and providing the critical details needed for ED personnel to provide care.
“With more than 100,000 people in the U.S. living with SCD, finding an intervention to help improve the speed and quality of ED care would be hugely impactful,” said Regina Hartfield, President and CEO of SCDAA. “For this program, we’re leveraging Medicalert’s decades of experience providing medical information in emergencies – as well as MedicAlert’s visibility in the medical community – to help people living with sickle cell.”
MedicAlert Foundation is a leading nonprofit providing lifesaving medical ID and emergency response services for millions of people living with chronic health conditions.
“Since 1956, MedicAlert has been globally trusted by both emergency medical personnel and people living with serious health conditions,” stated Karen Cassel, MedicAlert Foundation’s President and CEO. “We’re pleased to launch this pilot during Sickle Cell Awareness Month. Our hope is to equip and empower SCD patients with tools to help them quickly get the care they need during a pain crisis.”
The pilot will run for one year. Two-hundred fifty adults in states with significant sickle cell populations are being recruited to participate in the first round.
“We believe this program has the potential to significantly improve outcomes for people experiencing sickle cell crises,” Hartfield said. “With positive results, we’ll seek additional funding to expand the program nationally.”
More details about the program, including full eligibility guidelines and how to apply, are available at medicalert.org/sicklecellpilot. Anyone living with SCD who has additional questions about the pilot should contact either their local SCDAA chapter, or email MedicAlert at sicklecellpilot@medicalert.org.
Abstracts Accepted Until 11:59 p.m. PST
This morning, we were notified that some users experienced issues using our 2023 National Abstract Competition portal. This issue has been remedied. SCDAA is accepting abstract submissions until 11:59 p.m. PST, July 17, 2023. If you tried to submit an abstract earlier today and encountered a problem, please click here to resubmit your work before the deadline. Thank you for your patience.
CMO Speaks: Gene Therapy for SCD (Part 1)

CMO Speaks is a blog featuring the voices of SCDAA’s clinical leadership team. Part 1 of our Gene Therapy series was written by Dr. Lewis Hsu and Dr. Sri Lakshmi Jamalapur.
How can you cure an inherited disease like sickle cell disease? One way would be to develop some extremely effective medications that would alleviate all the symptoms. Another would be to transplant stem cells that produce red blood cells. But the ultimate way to cure genetic disease is with gene therapy.
Gene therapy is a relatively new treatment for sickle cell warriors. Researchers began conducting experimental studies (clinical research) in people about seven years ago. At first, the only approach to this treatment was a process called “gene addition” developed by the Boston-based pharmaceutical company bluebird bio. Soon, several other groups joined with other approaches for gene therapy. By early 2022, there were six competing organizations enrolling in gene therapy clinical research. They are listed in the table below.
There has been a lot of news recently regarding gene therapy. Earlier this year, The New York Times published this article about gene therapy and sickle cell disease, which highlighted the voices and experiences of advocates from around our community and brought renewed attention to this pioneering treatment. Two pharmaceutical groups say that they will bring their gene therapy clinical research results to the U.S. Food and Drug Administration (FDA) for possible approval as safe and effective in the first quarter of 2023 (highlighted green in the table).
At the same time, in late February, three other groups announced that they are closing their gene therapy clinical research studies (highlighted yellow in the table). Some people worry that seeing companies end their gene therapy studies is not good news for sickle cell disease. However, another way to look at these announcements is that the most fruitful studies are heading toward possible FDA approval. Less productive clinical research efforts are cutting their losses and being pruned away.
It’s like a gardener doing work in a little garden to keep the plants that are bearing fruit (the two groups that will bring their results to U.S. FDA) alive but removing those plants that have stopped growing (the groups that are closing their gene therapy clinical research). The wisdom of pruning down the less productive activity is recognized by many proverbs, including “if you run after two hares, you will catch neither,” or “have too many irons in the fire,” or “he who tries to sit on two chairs will end up sitting on neither,” or John 15:1 in the Bible. Drop-out studies are part of the healthy competitive process of research and investment.
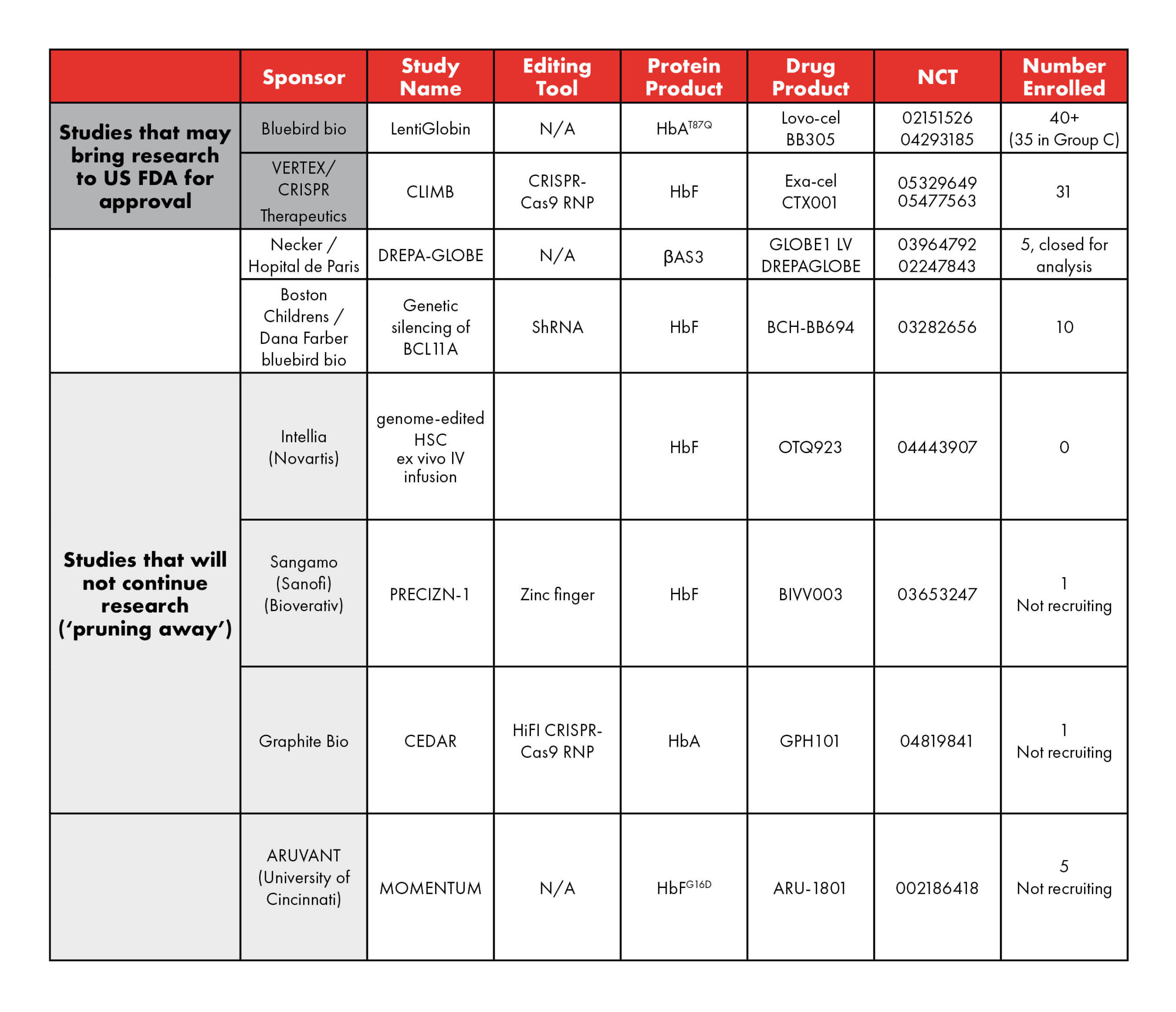
Table 1: Current and upcoming studies of gene therapy in SCD. (adapted from Kanter and Falcon 2021; Hsu and Jamalapur 2022) N/A – not applicable; LVV – lentiviral vector; HbAT87Q – Hb with a single mutation conferring most of the antisickling effect of γ-globin; βAS3 – an antisickling β-globin containing 3 amino acid substitutions in the wild-type HBB; HbFG16D – modified HbF that increases affinity to α-globin to outcompete sickle mutated β-globin; AAV6 – Adeno-Associated Virus serotype 6.
Outside of gene therapy studies, promising sickle cell progress is everywhere. Researchers are developing innovative medications and studying stem cell transplants that could make a world of difference to warriors who are unable or unwilling to undergo gene therapy in the future. The clinical research process is fine-tuned to determine which treatments are safe and effective. Sickle cell warriors are encouraged to help push this research forward by participating in clinical trials.
Lewis Hsu, MD, PhD, is a pediatric hematologist who serves as director of the Sickle Cell Center and professor of pediatrics for the University of Illinois at Chicago. He has conducted sickle cell research, published over 50 peer-reviewed papers and co-authored “Hope and Destiny: The Patient and Parent’s Guide to Sickle Cell Disease and Sickle Cell Trait.” He currently serves as the SCDAA Chief Medical Officer.
Sri Lakshmi Jamalapur, MD, is a pediatric hematologist and the new leader for the Pediatric Sickle Cell Center at Children’s Hospital of Michigan in Detroit. She has collaborated with Dr. Hsu on health education and sickle cell advocacy since 2019.
June 19, 2023, is Officially Sickle Cell Awareness Day in Maryland!
 Maryland Governor Wes Moore has signed a proclamation to make June 19, 2023, Sickle Cell Awareness Day! This recognition goes a long way in raising awareness about sickle cell disease, combating prejudices and lifting up our community. Thank you for supporting our cause and helping us to “Shine the Light on Sickle Cell Disease!”
Maryland Governor Wes Moore has signed a proclamation to make June 19, 2023, Sickle Cell Awareness Day! This recognition goes a long way in raising awareness about sickle cell disease, combating prejudices and lifting up our community. Thank you for supporting our cause and helping us to “Shine the Light on Sickle Cell Disease!”
SCDAA hires member coordinator
 The Sickle Cell Disease Association of America, a national nonprofit membership organization that advocates for people affected by sickle cell disease, named Kristen Cox as member engagement coordinator. The association has more than 50 member organizations throughout the United States.
The Sickle Cell Disease Association of America, a national nonprofit membership organization that advocates for people affected by sickle cell disease, named Kristen Cox as member engagement coordinator. The association has more than 50 member organizations throughout the United States.
Prior to joining the Sickle Cell Disease Association of America, Cox served as the membership and leadership manager for the American Society of Consultant Pharmacists for five years, where she used her extensive experience in management and member cultivation to build relationships with the society’s over 1,000 members.
Previously, she served at the DiLorenzo Tricare Health Clinic at the Pentagon in Washington, D.C., where she advanced her administrative and customer service skills. Cox began her career as a certified nursing assistant.
She holds a master’s degree from the University of Maryland School of Pharmacy and bachelor’s degree from the University of Maryland.
Women’s History Month: Dr. Marilyn Hughes Gaston
This #WomensHistoryMonth, we are highlighting prominent women who have made lasting contributions to the SCD community. #WomenInMedicine #WomensHistoryMonth

Meet Dr. Marilyn Hughes Gaston, an internationally recognized leader in health care equality and sickle cell disease advocacy. Since 1976, she has dedicated her career to improving medical care for poor and minority families and has contributed to significant changes in the management of sickle cell disease. Despite facing racial and economic barriers, Dr. Gaston made history by publishing a groundbreaking study on sickle cell disease that led to a nationwide screening program for newborns. This initiative resulted in a significant reduction of morbidity and mortality in young children with the disease around the world. Let us acknowledge Dr. Gaston’s invaluable contributions to public health and unwavering commitment to bridging the gap of health disparities for all Americans.
Women’s History Month: Dr. Yvette Francis-McBarnette
This #WomensHistoryMonth, we are highlighting prominent women who have made lasting contributions to the SCD community. #WomenInMedicine #WomensHistoryMonth
 Meet Dr. Yvette Francis-McBarnette, a Jamaican-born physician who specialized in treating children with sickle cell anemia. As one of the first Black women to graduate from the Yale School of Medicine, Dr. Francis-McBarnette was credited with successfully using antibiotics to treat children with sickle cell anemia 15 years before the effectiveness of those drugs were confirmed. Dr. Francis-McBarnette was also part of the White House advisory committee which made recommendations that led to the 1972 National Sickle Cell Anemia Control Act. Her contributions continue to inspire and impact the sickle cell community today.
Meet Dr. Yvette Francis-McBarnette, a Jamaican-born physician who specialized in treating children with sickle cell anemia. As one of the first Black women to graduate from the Yale School of Medicine, Dr. Francis-McBarnette was credited with successfully using antibiotics to treat children with sickle cell anemia 15 years before the effectiveness of those drugs were confirmed. Dr. Francis-McBarnette was also part of the White House advisory committee which made recommendations that led to the 1972 National Sickle Cell Anemia Control Act. Her contributions continue to inspire and impact the sickle cell community today.
Women’s History Month: Dr. Helen M. Ranney
This #WomensHistoryMonth, we are highlighting prominent women who have made lasting contributions to the SCD community. #WomenInMedicine #WomensHistoryMonth

Meet Dr. Helen M. Ranney, physician and hematologist. Born in 1920, Dr. Ranney dedicated her work to researching blood disorders. Her groundbreaking work on sickle cell anemia included the first description of abnormal blood cell structure and genetic factors, earning her the Dr. Martin Luther King Jr. Medical Achievement Award in 1972. Dr. Ranney was also the first woman president of the Association of American Physicians. Let’s take a moment to celebrate Dr. Ranney for the work she has done on behalf of the sickle cell community!
Women’s History Month: Dr. Angella Dorothea Ferguson
This #WomensHistoryMonth, we are highlighting prominent women who have made lasting contributions to the SCD community. #WomenInMedicine #WomensHistoryMonth

Meet Dr. Angella Dorothea Ferguson, a pediatrician and sickle cell pioneer. Born in 1925, Dr. Ferguson dedicated her life to researching sickle cell disease, an unknown condition at the time. Her groundbreaking work led to the development of a blood test for infants, which is now the standard in most states. Thanks to her research, we better understand which symptoms to look for in children and can start treating sickle cell earlier. Thanks to Dr. Ferguson for the work she has done on behalf of our community!





 DONATE NOW
DONATE NOW