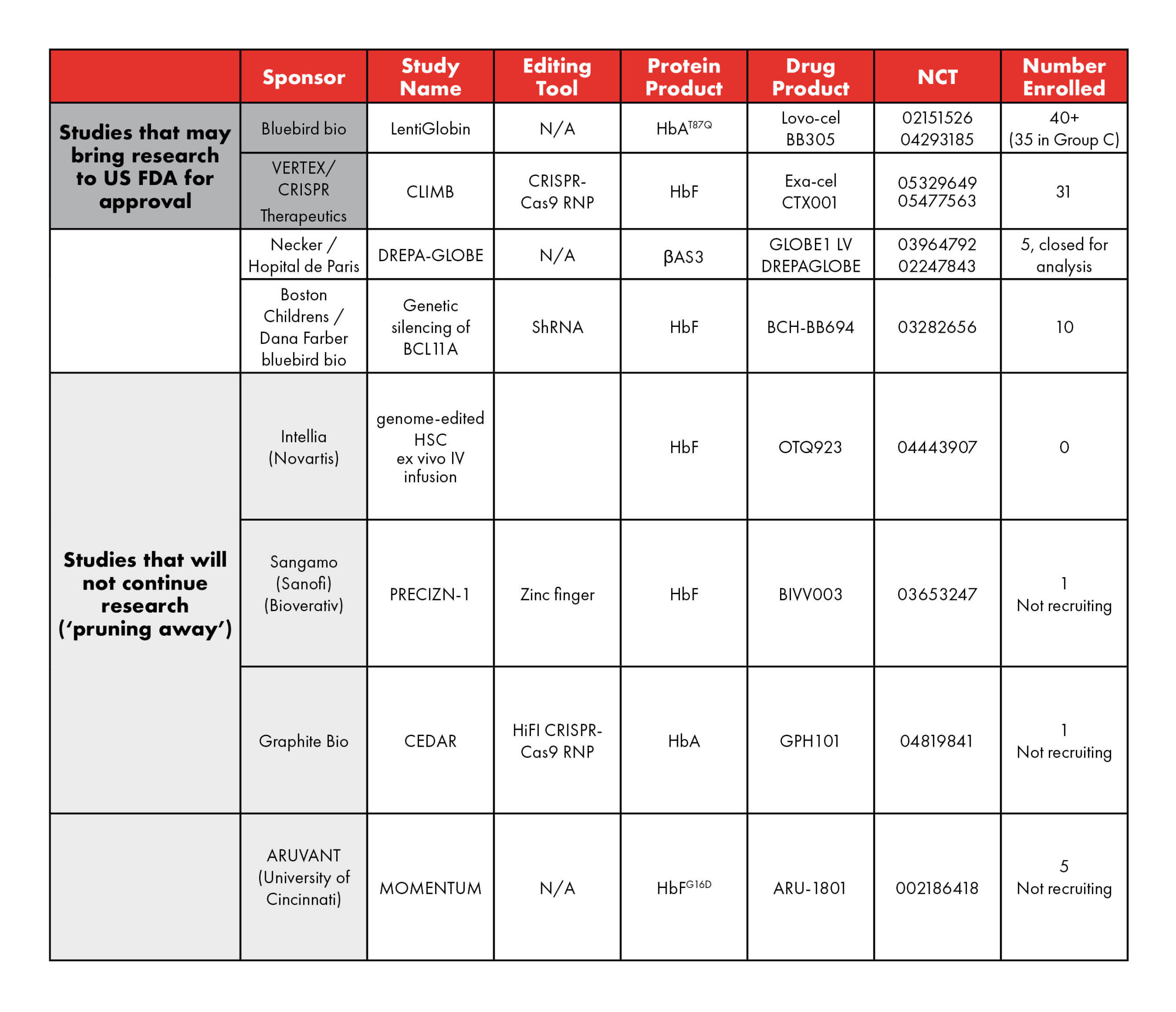
Dublin, Ireland, 19 June 2023: Afimmune, a clinical stage biopharmaceutical company developing novel rare disease therapeutics, today announced it has been invited to join the Sickle Cell Disease Association of America’s (SCDAA) Collaboration of Advocates for Research, Education and Science (C.A.R.E.S.) Consortium.
The mission of this initiative is to raise awareness about the importance of clinical trials and why it can help sickle cell patients to participate in them. The SCDAA along with other participating strategic pharma partners came together to help educate sickle cell patients on the potential treatment options available and to encourage more clinical trial participation.
Commenting on the update, Dr. Moayed Hamza, MD, Chief Medical Officer of Afimmune said,“With today being World Sickle Cell Day, it is timely to announce our membership to the SCDAA C.A.R.E.S. Consortium. I look forward to working with the other members to continue to drive improvement in the treatment landscape for patients suffering from sickle cell disease.”
SCDAA President and CEO, Ms. Regina Hartfield said, “We are thrilled that Afimmune has joined our expanding consortium and look forward to collaborating with their team to increase awareness within the sickle cell community.”
About Epeleuton
Epeleuton is 15-hydroxy eicosapentaenoic acid (15(S)-HEPE) ethyl ester, a novel synthetic fatty acid drug product. Epeleuton has been shown to have a unique dual mechanism of action for the treatment of SCD, targeting factors affecting severity, course of disease, and vaso-occlusive crisis risk.
Afimmune is developing Epeleuton for SCD due to its novel disease-modifying preclinical efficacy, first-in-class opportunity and a significantly reduced regulatory pathway. Epeleuton has received orphan drug designation for the treatment of SCD from the FDA and EMA
About Sickle Cell Disease
SCD is a group of inherited, progressive blood disorders carried by the β allele of the hemoglobin gene with an expected 30-year reduced life expectancy. The disease is characterized by abnormal polymerization of hemoglobin during oxygenation which results in the sickling of red blood cells. The disease is rare, with an estimated prevalence of only ~100,000 people affected in the US and ~52,000 people in the EU.
About Afimmune
Afimmune, headquartered in Dublin, Ireland, is a clinical stage drug discovery and development company working on new medicines to improve the quality of life for people with rare and inflammatory diseases.
About SCDAA
Sickle Cell Disease Association of America advocates for people affected by sickle cell conditions and empowers community-based organizations to maximize quality of life and raise public consciousness while advancing the search for a universal cure. The association and more than 50 member organizations support sickle cell research, public and professional health education and patient and community services. (www.sicklecelldisease.org)
Contact
investor.relations@afimmune.com






 Maryland Governor Wes Moore has signed a proclamation to make June 19, 2023, Sickle Cell Awareness Day! This recognition goes a long way in raising awareness about sickle cell disease, combating prejudices and lifting up our community. Thank you for supporting our cause and helping us to “Shine the Light on Sickle Cell Disease!”
Maryland Governor Wes Moore has signed a proclamation to make June 19, 2023, Sickle Cell Awareness Day! This recognition goes a long way in raising awareness about sickle cell disease, combating prejudices and lifting up our community. Thank you for supporting our cause and helping us to “Shine the Light on Sickle Cell Disease!”  The Sickle Cell Disease Association of America, a national nonprofit membership organization that advocates for people affected by sickle cell disease, named Kristen Cox as member engagement coordinator. The association has more than 50 member organizations throughout the United States.
The Sickle Cell Disease Association of America, a national nonprofit membership organization that advocates for people affected by sickle cell disease, named Kristen Cox as member engagement coordinator. The association has more than 50 member organizations throughout the United States.



 Meet Dr. Yvette Francis-McBarnette, a Jamaican-born physician who specialized in treating children with sickle cell anemia. As one of the first Black women to graduate from the Yale School of Medicine, Dr. Francis-McBarnette was credited with successfully using antibiotics to treat children with sickle cell anemia 15 years before the effectiveness of those drugs were confirmed. Dr. Francis-McBarnette was also part of the White House advisory committee which made recommendations that led to the 1972 National Sickle Cell Anemia Control Act. Her contributions continue to inspire and impact the sickle cell community today.
Meet Dr. Yvette Francis-McBarnette, a Jamaican-born physician who specialized in treating children with sickle cell anemia. As one of the first Black women to graduate from the Yale School of Medicine, Dr. Francis-McBarnette was credited with successfully using antibiotics to treat children with sickle cell anemia 15 years before the effectiveness of those drugs were confirmed. Dr. Francis-McBarnette was also part of the White House advisory committee which made recommendations that led to the 1972 National Sickle Cell Anemia Control Act. Her contributions continue to inspire and impact the sickle cell community today.